Cosmetic packaging is far more than an aesthetic choice—it’s a vital intersection of marketing strategy, product safety, and legal compliance. In today’s increasingly globalized beauty industry, packaging must do more than attract the eye; it must also protect the product, convey essential information, and meet complex regulatory standards that vary dramatically across regions. As international beauty brands continue to scale their presence across borders, understanding and navigating the intricate landscape of cosmetic packaging regulations has become not only important but imperative.
This blog provides a comprehensive, country-by-country breakdown of current cosmetic packaging regulations as of June, 2025. It highlights the unique requirements of major markets, notes recent legislative updates, and explores emerging trends such as eco-friendly mandates, QR-code labeling, and the integration of AI in traceability and compliance monitoring. Whether you’re a multinational corporation or a rising indie brand, this guide is designed to help you anticipate regulatory hurdles, avoid costly delays, and ensure that your packaging meets both consumer expectations and legal obligations in every market you serve.
The Role of Packaging Regulations in Cosmetics
Definition and Scope
Cosmetic packaging regulations govern the design, materials, labeling, and presentation of cosmetic products. These rules are not just about aesthetics; they protect consumers, ensure product integrity, and facilitate international trade. Packaging regulations address:
- Safety: Preventing contamination, ensuring materials are non-reactive, and maintaining product stability.
- Transparency: Mandating clear, truthful information about ingredients, usage, and manufacturer.
- Environmental Impact: Encouraging or requiring recyclable, sustainable materials and responsible waste management.
- Market Access:Setting standards that, if met, allow products to be sold legally in a given market.
Common Regulatory Themes
- Safety Assessments: All packaging materials must be proven safe for use with cosmetics, ensuring no harmful substances leach into products.
- Labeling Requirements: Labels must clearly state product identity, ingredients, warnings, manufacturer/importer details, and other mandated information.
- Environmental Standards: Increasingly, regulations require recyclable materials, limit certain plastics, and promote circular economy approaches.
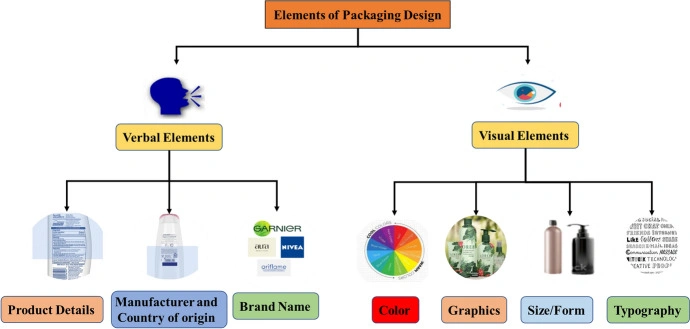
China: CSAR, NMPA, and New Labeling Rules
Regulatory Framework
China’s Cosmetics Supervision and Administration Regulation (CSAR) and the Administrative Measures on Cosmetics Labelling set strict standards for packaging and labeling. The National Medical Products Administration (NMPA) is the main regulatory authority.
Packaging and Labeling Requirements
- Chinese Labeling: All products must have labels in standard Chinese characters. Exceptions are made for trade names, websites, and foreign company addresses.
- Ingredient Listing: Ingredients above 0.1% must be listed in descending order; those below 0.1% are grouped as “other trace ingredients”.
- Mandatory Information: Product name, manufacturer, shelf life, warnings, executive standard number (registration or notification code), and efficacy claims must be present.
- Prohibited Claims: Labels cannot use medical terms, guarantee safety/efficacy, or make false/exaggerated claims.
- Ingredient Consistency: Any ingredient or efficacy claim must match the product’s actual formula.
- Testing: All imported cosmetics must pass NMPA-designated laboratory tests for safety and efficacy before market entry.
- Sample Retention: Companies must retain samples in original packaging for at least six months after expiry, and maintain traceable records of packaging materials.
- Children’s Cosmetics: Additional labeling and safety requirements apply to products intended for children.
Recent Regulatory Changes
China has unified and sharpened labeling requirements, introduced annual internal audits for Good Manufacturing Practice compliance, and strengthened traceability and post-market supervision. Packaging materials must not chemically interact with the product or release harmful substances.

United States: MoCRA and FDA
Regulatory Framework
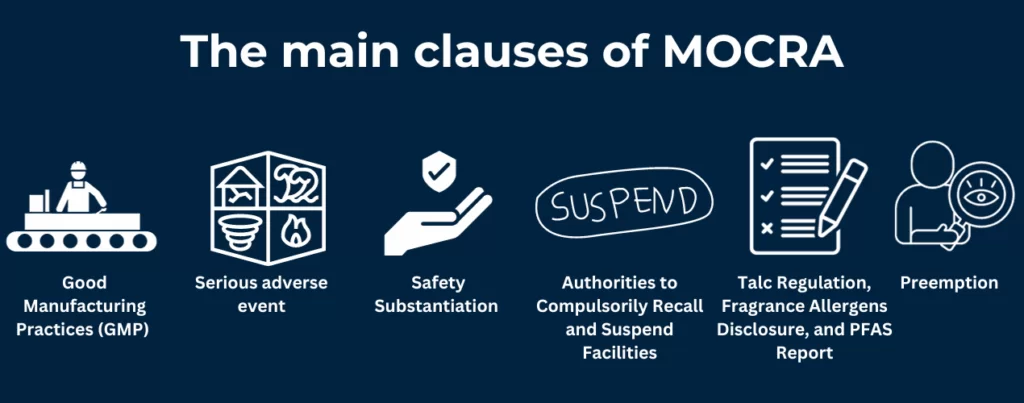
The U.S. Food and Drug Administration (FDA) oversees cosmetics under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act. The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) is the most significant update in over 80 years, introducing sweeping new requirements.
Key Packaging and Labeling Requirements
- Facility Registration: All domestic and foreign manufacturers must register with the FDA every two years. Changes must be reported within 60 days. Exemptions apply for very small businesses, except those making high-risk products.
- Product Listing: Every cosmetic product—including each fragrance, color, or seasonal variant—must be listed with the FDA, with full ingredient disclosure.
- Safety Substantiation: Companies must maintain scientific evidence (e.g., toxicity tests, expert reviews) proving each product’s safety under normal use.
- Adverse Event Reporting: Serious health incidents (hospitalization, disfigurement, etc.) must be reported to the FDA within 15 business days.
- Labeling Enhancements:
- Identity Statement: Clearly state the product’s identity (e.g., “shampoo”) on the principal display panel.
- Net Quantity: Indicate the amount (weight, measure, or count).
- Ingredient Declaration: List all ingredients in descending order of predominance, including fragrances and color additives.
- Name and Place of Business: Manufacturer, packer, or distributor must be named.
- Warnings and Directions: Include necessary warnings and usage directions.
- Fragrance Allergen Disclosure: MoCRA requires labeling of fragrance allergens (pending FDA’s final rule).
- Responsible Person: Label must include the responsible party’s contact info for consumer safety and adverse event reporting.
- Professional Use Labeling: Products for professionals must be clearly labeled as such.
Recent Developments & Challenges
The implementation of the Modernization of Cosmetics Regulation Act (MoCRA) in the United States has ushered in the most sweeping changes to cosmetic regulation in decades. For the first time, cosmetic manufacturers and brands are required to adhere to federally mandated standards for product safety, labeling, adverse event reporting, and facility registration. Among the most significant challenges introduced by MoCRA are its robust requirements for data collection, real-time reporting, and safety substantiation—factors that demand more than just good manufacturing practices; they require systemic change and strategic planning.
These regulatory shifts are especially burdensome for small and independent brands. While MoCRA includes exemptions for businesses generating less than \$1,000,000 in annual revenue (adjusted for inflation), these exemptions do not apply to certain product categories deemed high-risk, such as products intended for the eye area, long-lasting or permanent makeup, and items that include color additives not generally recognized as safe. As a result, many small businesses find themselves navigating a complex regulatory environment with limited resources.
At the same time, MoCRA is acting as a catalyst for innovation across the U.S. cosmetics market. The need for clearer ingredient labeling, validated safety assessments, and improved traceability is pushing brands toward cleaner formulations, transparent supply chains, and digital-first compliance strategies. From QR code-enabled packaging to AI-assisted product documentation, the pressure to meet regulatory demands is reshaping how cosmetics are developed, packaged, and marketed.
Still, meeting these expectations requires investment—in compliance infrastructure, scientific expertise, and technology platforms capable of handling regulatory reporting and safety substantiation at scale. For emerging brands aiming to grow, partnering with experienced contract manufacturers, regulatory consultants, or packaging specialists has become increasingly essential to ensure alignment with MoCRA’s evolving framework.
European Union: Regulation (EC) 1223/2009 and Sustainability
Regulatory Framework
The EU’s Regulation (EC) 1223/2009 is the gold standard for cosmetic regulation, harmonizing rules across all member states. The regulation covers safety, labeling, packaging, and environmental considerations, and is regularly updated to address new scientific findings (e.g., nanomaterials).
Packaging Safety Assessment
- Safety Report: Manufacturers must conduct a scientific safety assessment, including packaging compatibility, before market entry. Packaging materials must not compromise product safety.
- Nanomaterials: Recent amendments (Regulation (EU) 2024/858) restrict or ban certain nanomaterials, requiring risk assessments and clear labeling for those permitted.
Labeling Requirements
- Responsible Person: Name and address of the responsible party (manufacturer or importer) must be on the label.
- Country of Origin: For imports, the label must state “made in [country]”.
- Nominal Content: Displayed in grams or milliliters, with exceptions for very small packages, samples, or multi-packs.
- Batch Number: Required for traceability; may appear only on outer packaging if space is limited.
- Function: If not obvious, the intended use must be stated in a language understood by the consumer.
- Date of Minimum Durability/PAO: Expiry date or Period After Opening (PAO) symbol for products with a shelf life over 30 months.
- Precautions and Warnings: Usage instructions and safety warnings, especially for products with specific risks (e.g., sunscreens, hair dyes).
- Ingredient List: Ingredients listed in descending order by weight, using INCI names; allergens and certain preservatives must be highlighted.
Sustainability and Environmental Focus
The EU leads in eco-packaging, promoting recyclable materials, bans on single-use plastics, and Extended Producer Responsibility (EPR) systems. Manufacturers are responsible for the entire lifecycle of packaging, and new rules (e.g., the Packaging and Packaging Waste Regulation, PPWR) further tighten sustainability requirements.

Japan: PMD Act and MHLW
Regulatory Framework
Japan regulates cosmetics under the Pharmaceuticals and Medical Devices (PMD) Act, administered by the Ministry of Health, Labour and Welfare (MHLW). The system distinguishes between cosmetics and quasi-drugs, with stricter requirements for the latter.
Packaging and Labeling Requirements
- Label Content:
1.Name and address of importer/distributor.
2.Brand name and product name by type.
3.Manufacturing number or code for traceability.
4.List of ingredients in Japanese, in descending order by quantity (using INCI names translated into Japanese).
5.Expiration date (if required by MHLW).
6.Country of origin.
7.Content (weight or capacity).
8.Precautions for usage/storage.
9.Information contact details.
- Material Identification: Packaging must display material identifiers (e.g., for paper or plastic) to promote recycling.
- Claims and Advertising: Prohibited from using exaggerated or misleading claims. The Consumer Affairs Agency (CAA) may require documentation for any claims of superior quality.
- Import and Licensing: Importers must hold the appropriate licenses and designate a Marketing Authorization Holder (MAH) responsible for regulatory compliance and quality management.
Environmental Focus
Japan’s approach to sustainable packaging is anchored by the Law for Promotion of Sorted Collection and Recycling of Containers and Packaging. This law plays a pivotal role in advancing recycling initiatives and reducing environmental impact across industries, including cosmetics.
- Material Identification Marks:
Under this law, all packaging—including cosmetic containers—must display clear marks indicating the type of material used, such as paper, plastic, glass, or metal. These identification marks are essential for facilitating the sorting and recycling of waste by consumers and municipal systems.
- Supporting Recycling Initiatives:
The primary aim of the law is to promote active consumer participation in waste sorting. By making packaging materials easily identifiable, the law encourages proper disposal and significantly improves recycling rates. This system supports Japan’s broader environmental goals and helps reduce landfill waste.
- Scope and Implementation:
The law applies to a wide range of packaging types, ensuring that manufacturers and importers of cosmetic products comply with the marking requirements. It is the responsibility of these companies to ensure that their packaging is appropriately labeled before products reach consumers.
- Recycling System:
Japan’s recycling system relies on consumers sorting packaging waste based on the material identification marks. This enables efficient collection and processing, making the recycling of cosmetic packaging more effective and sustainable.
In summary, Japan’s legal framework for packaging not only mandates material transparency but also fosters a culture of recycling and environmental stewardship, setting a strong example for sustainable packaging practices in the cosmetics industry.

India: Drugs and Cosmetics Act, CDSCO, and BIS
Regulatory Framework
India’s Drugs and Cosmetics Act, Cosmetic Rules 2020, and the Bureau of Indian Standards (BIS) regulate cosmetic packaging and labeling. The Central Drugs Standard Control Organisation (CDSCO) is the main authority.
Packaging and Labeling Requirements
- Language: Labels must be in English or Hindi, with legible font size and style.
- Mandatory Information:
1.Name of cosmetic product.
2.Name and address of manufacturer (and importer, if applicable).
3.Manufacturing and expiry dates.
4.Ingredient list in descending order.
5.Batch number (for solids/semi-solids above 10g, liquids above 25ml).
6.Net content (weight/volume for liquids above 60ml, solids/semi-solids above 30g).
7.Warnings and precautions (e.g., “For external use only”).
8.Declaration of hazardous/special-use ingredients, with name and quantity.
9.Country of origin for imports.
- Label Integrity: Labels must be securely affixed and not altered or erased after application.
- BIS Standards: Additional packaging and labeling standards apply for specific cosmetic categories under BIS guidelines.
South Korea: Cosmetics Act, MFDS, and Recycling
Regulatory Framework
South Korea’s Cosmetics Act, enforced by the Ministry of Food and Drug Safety (MFDS), sets high standards for packaging safety, labeling, and sustainability.
Packaging and Labeling Requirements
- Label Content:
1.Name and address of manufacturer and seller.
2.Product name, batch number, content, and shelf life (including after opening).
3.Full ingredient list.
4.Precautions for use and warnings.
5.Price and functional claims, if applicable.
- Material Restrictions: Use of PVC and colored PET bottles is banned; packaging materials must be graded for recyclability (“best to recycle” to “difficult to recycle”).
- Recycling Symbols: Packaging must display recycling grades and symbols. If packaging is “difficult to recycle,” it must be labeled as such unless the company participates in a reverse recycling scheme.
- Reverse Recycling Scheme: Companies can collect used containers from consumers and may be exempted from the “difficult to recycle” label if they have an approved recycling plan.
- Child Safety: Certain products require child-resistant packaging to prevent accidental ingestion or misuse.
Recent Developments
South Korea has become a leader in sustainable cosmetic packaging by implementing strict regulations and guidelines focused on recyclability and eco-friendliness, driven by both consumer demand and government initiatives. The Ministry of Food and Drug Safety (MFDS) and the Ministry of Environment require all cosmetic packaging to display recyclability grades and standardized recycling symbols, ban the use of difficult-to-recycle materials like PVC and colored PET, and encourage packaging minimization and participation in take-back programs. These efforts have spurred innovation among brands, leading to the adoption of refillable, biodegradable, and recycled packaging solutions, and are positioning South Korea as a global benchmark for environmentally responsible beauty packaging.

Expanded Topics in Cosmetic Packaging Regulations
Smart Packaging and Digital Innovation
Digital technology is transforming cosmetic packaging into an interactive and intelligent interface.
Smart packaging leverages tools like NFC (Near Field Communication) tags, QR codes, RFID chips, and even embedded sensors. These features allow consumers to scan packaging with their smartphones to access authenticity verification, product tutorials, ingredient breakdowns, and even personalized skincare recommendations.
For example, a QR code on a serum bottle might link to a video on proper application, while an NFC tag could confirm the product is genuine and not counterfeit.
Airless dispensing systems are another innovation, protecting sensitive formulations from air and contamination, while self-heating tubes and personalized dispensers use embedded tech to deliver tailored doses or activate ingredients on demand.
These advancements not only improve regulatory compliance (by simplifying traceability and recall procedures) but also deepen consumer engagement and loyalty.
Sustainable and Zero-Waste Packaging
Sustainability is now at the heart of cosmetic packaging innovation, driven by both consumer demand and regulatory pressure.
Brands are increasingly adopting biodegradable, compostable, and recyclable materials—such as bamboo, cornstarch, mushroom-based bioplastics, and recycled glass or aluminum.
Refillable packaging is gaining momentum, with brands offering refill pouches or in-store refill stations to reduce single-use plastic waste.
Closed-loop recycling programs—where brands collect and recycle used packaging—are being pioneered by leaders like Lush and RMS Beauty, who use post-consumer recycled (PCR) materials in their packaging lines.
Regulators in the EU and South Korea are setting ambitious targets for recyclability and recycled content, influencing global trends and raising the bar for eco-friendly packaging.
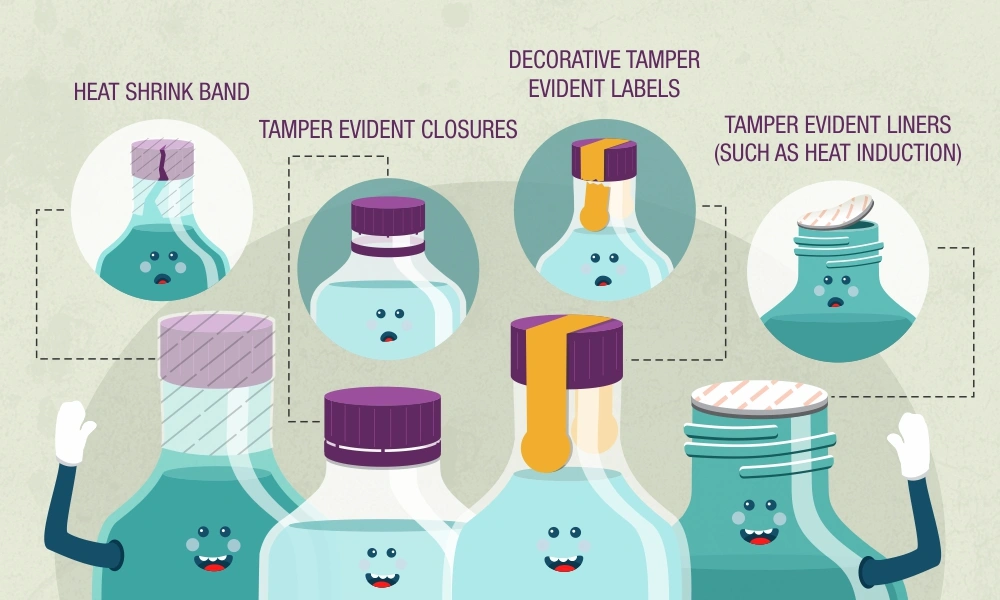
Tamper-Evident and Safety Features
Tamper-evident packaging is now a cornerstone of consumer protection in the cosmetics industry. Regulatory agencies in the US, EU, and many other jurisdictions either mandate or strongly recommend the use of tamper-evident features to prevent product adulteration and reassure consumers of product integrity.
Common tamper-evident mechanisms include shrink bands, induction seals, breakable caps, and tamper-proof stickers. The US FDA, for example, provides guidance on acceptable tamper-evident packaging for over-the-counter (OTC) drugs and cosmetics, while the EU’s General Product Safety Directive encourages similar approaches for consumer goods.
Brands are also innovating with aesthetic and user-friendly solutions—for instance, integrating tamper-evident seals into the design of luxury jars or using color-changing indicators that reveal if a package has been opened.
Consumer education is increasingly important: brands often include icons or instructions on how to check for tampering, which not only enhances safety but also builds trust and brand reputation.
Design Considerations and Accessibility
Cosmetic packaging must balance regulatory compliance with branding and user experience.
Design requirements often specify minimum font size, contrast, and the placement of mandatory information to ensure legibility for all consumers.
Accessibility is an emerging focus, with easy-to-open, ergonomic packaging designed for elderly consumers or those with disabilities. Some jurisdictions require tactile warnings (such as raised symbols for hazardous products) or Braille labeling for vision-impaired users, especially on products like hair dyes or nail polish removers.
Brands are also exploring universal design principles—creating packaging that is intuitive and usable by the widest possible audience, without sacrificing aesthetics or brand identity.
Recycling and Multilayer Packaging Challenges
Multilayer packaging—which combines different materials to improve product protection and shelf life—poses significant recycling challenges, as it is difficult to separate layers for processing.
To address this, the EU and South Korea are pushing for mono-material packaging (using a single recyclable material) and providing incentives for brands to simplify packaging structures.
Design-for-recycling is a growing trend, with companies rethinking adhesives, inks, and coatings to ensure full recyclability.
Advanced recycling technologies—such as chemical recycling, upcycling, and microbial degradation—are being explored to handle complex packaging waste.
Regulatory frameworks are evolving to reward innovation in this space, with extended producer responsibility (EPR) schemes holding brands accountable for the end-of-life management of their packaging.
Allergen and Ingredient Transparency
Transparency in ingredient disclosure is a top regulatory priority worldwide.
The EU requires the declaration of 26 specific fragrance allergens on cosmetic labels if present above certain thresholds, while the US is moving toward similar requirements under the Modernization of Cosmetics Regulation Act (MoCRA).
Ingredient lists must be clear, accurate, and presented in a prescribed order (usually descending by weight).
There is also a trend toward full ingredient disclosure—including the source and function of each ingredient—driven by consumer demand for “clean beauty” and regulatory updates.
Lists of restricted and prohibited substances are regularly updated, and brands must stay vigilant to ensure ongoing compliance.
Emerging Trends: Minimalism, Multipurpose, and Clean Beauty
Minimalist packaging—using fewer materials, less ink, and simpler designs—is gaining popularity for both sustainability and aesthetic reasons.
Multipurpose cosmetics (e.g., a lip and cheek tint in one) reduce the need for multiple products and excessive packaging, appealing to eco-conscious and convenience-driven consumers.
The clean beauty movement is influencing packaging choices, with greater scrutiny on material safety, recyclability, and the avoidance of harmful chemicals.
Regulators are responding by tightening rules on packaging claims and requiring substantiation for terms like “natural,” “organic,” or “non-toxic.”

Nanotechnology and Advanced Materials
Nanomaterials are increasingly used in cosmetic packaging for their superior barrier properties, lightweight strength, and ability to preserve product freshness.
However, their use is under regulatory scrutiny—especially in the EU—due to concerns about potential health and environmental risks.
Brands must provide safety data and comply with evolving labeling requirements for nanomaterials, while also leveraging their benefits for innovative, high-performance packaging.
Global Harmonization and International Trade
As cosmetic brands expand internationally, they face a complex web of overlapping and sometimes conflicting packaging regulations.
Efforts toward global harmonization—such as the adoption of ISO 22715 standards and mutual recognition agreements—are ongoing, but significant differences remain in areas like language requirements, allergen disclosure, and material restrictions.
Export packaging must often be customized to meet both the origin and destination country’s rules, particularly regarding labeling, safety features, and environmental compliance.
Staying abreast of these evolving requirements is essential for brands seeking seamless global market access.
In summary:
The regulatory landscape for cosmetic packaging is rapidly evolving, with a strong focus on consumer safety, transparency, digital innovation, sustainability, and accessibility. Brands that proactively address these expanded topics—by investing in tamper-evident features, smart packaging, sustainable materials, inclusive design, and global compliance—will not only meet regulatory expectations but also build lasting consumer trust and competitive advantage in the global beauty market.
Challenges and Trends in Cosmetic Packaging Regulation
Globalization and Harmonization
As cosmetic brands expand into new markets, they must navigate a complex landscape of regulatory requirements that often differ significantly across regions. For example, ingredient disclosure, language, packaging material restrictions, and labeling formats can vary between the EU, US, China, and other major markets. While initiatives such as the adoption of ISO 22715 (international standards for cosmetic labeling and packaging) are fostering some degree of harmonization, substantial differences remain. This lack of uniformity means brands often need to create region-specific packaging, increasing costs and operational complexity. Staying compliant requires ongoing monitoring of regulatory updates and a flexible, well-informed approach to packaging design and supply chain management.
Sustainability
Sustainability has shifted from a trend to a baseline expectation for both regulators and consumers. The EU is leading the way with binding recycled content targets, bans on single-use plastics, and Extended Producer Responsibility (EPR) schemes that make brands accountable for the entire lifecycle of their packaging. South Korea and Japan are also advancing sustainability through strict recyclability standards and mandatory material identification marks. In response, brands are investing in mono-material packaging (which simplifies recycling), refillable systems, compostable and biodegradable materials, and closed-loop recycling programs. These efforts not only reduce environmental impact but also align with evolving regulatory and consumer demands.
Digitalization
The rise of digital technologies is transforming cosmetic packaging. Smart packaging solutions—such as QR codes, NFC tags, and RFID chips—are being used for regulatory compliance, digital batch tracking, product authentication, and interactive consumer engagement. These technologies enable brands to provide detailed product information, usage tutorials, and safety alerts, while also improving traceability and facilitating recalls if necessary. Digitalization supports transparency and can enhance consumer trust, making it a valuable tool in both regulatory compliance and marketing.
Future Outlook
Looking ahead, cosmetic packaging regulations are expected to become even more stringent, particularly in the areas of sustainability, transparency, and consumer safety. Governments are likely to introduce tougher requirements for recycled content, eco-design, and digital traceability, while also demanding greater ingredient and allergen transparency. Brands that anticipate these changes—by investing in sustainable materials, robust safety validation, and digital innovation—will not only ensure compliance but also strengthen their competitive position in the global market. Proactive adaptation, continuous regulatory monitoring, and a commitment to responsible packaging will be essential for long-term success in the evolving beauty industry.

Consumer and NGO Influence
Consumer advocacy groups and environmental NGOs play a pivotal role in shaping cosmetic packaging regulations worldwide. These organizations often act as watchdogs, pushing for greater transparency, stricter safety standards, and more sustainable practices within the beauty industry. For example, groups like Breast Cancer Prevention Partners and the Environmental Working Group in the United States have conducted independent research, published ingredient safety databases, and lobbied for legislative reforms to restrict harmful chemicals and require clearer labeling. Their efforts have contributed to landmark regulatory changes, such as the passage of the Modernization of Cosmetics Regulation Act (MoCRA), by raising public awareness and keeping pressure on lawmakers to prioritize consumer health and environmental protection.
NGOs and advocacy groups also frequently engage in public education campaigns, helping consumers understand ingredient labels, identify potential risks, and make informed purchasing decisions. Their independent testing and reporting can uncover safety issues not yet addressed by regulators or industry, further driving improvements in packaging safety and sustainability.
To support consumer protection, many regions have established public reporting mechanisms that empower individuals to report adverse reactions, quality concerns, or labeling violations directly to authorities. In the United States, for instance, the FDA offers multiple channels—including MedWatch online forms, hotlines, and dedicated contact centers—for consumers and health professionals to report cosmetic product issues such as allergic reactions, contamination, or misleading packaging. These reports can trigger regulatory investigations, product recalls, or further scrutiny of industry practices. Some advocacy organizations also provide their own reporting tools or partner with brands to facilitate consumer feedback and adverse event tracking.
Overall, the active involvement of consumers and NGOs not only drives regulatory evolution but also increases accountability and transparency within the cosmetics industry. Their influence ensures that packaging regulations continue to reflect public health priorities, environmental concerns, and the demand for ethical business practices.
Conclusion
Cosmetic packaging regulations are a cornerstone of product safety, transparency, and environmental responsibility in the modern beauty industry. As regulations evolve and diverge across regions, brands are challenged to navigate a complex and ever-changing global landscape. Staying informed about the latest requirements in each key market—whether related to labeling, material safety, sustainability, or digital innovation—is essential not only for legal compliance but also for maintaining consumer trust and brand reputation.
Ultimately, brands that embrace regulatory rigor, prioritize eco-friendly and accessible packaging, and proactively adapt to emerging trends will be best positioned for long-term growth and leadership in the competitive world of cosmetics. By making regulatory compliance a foundation of their business strategy, companies can deliver safer, more transparent, and more sustainable products that resonate with today’s conscious consumers, ensuring both market success and a positive impact on society and the environment.
This article is based on the most current regulations and trends as of June 2025, drawing from authoritative regulatory sources and recent updates.



